
Optimi Health announces the launch of a psilocybin product
by CM Staff
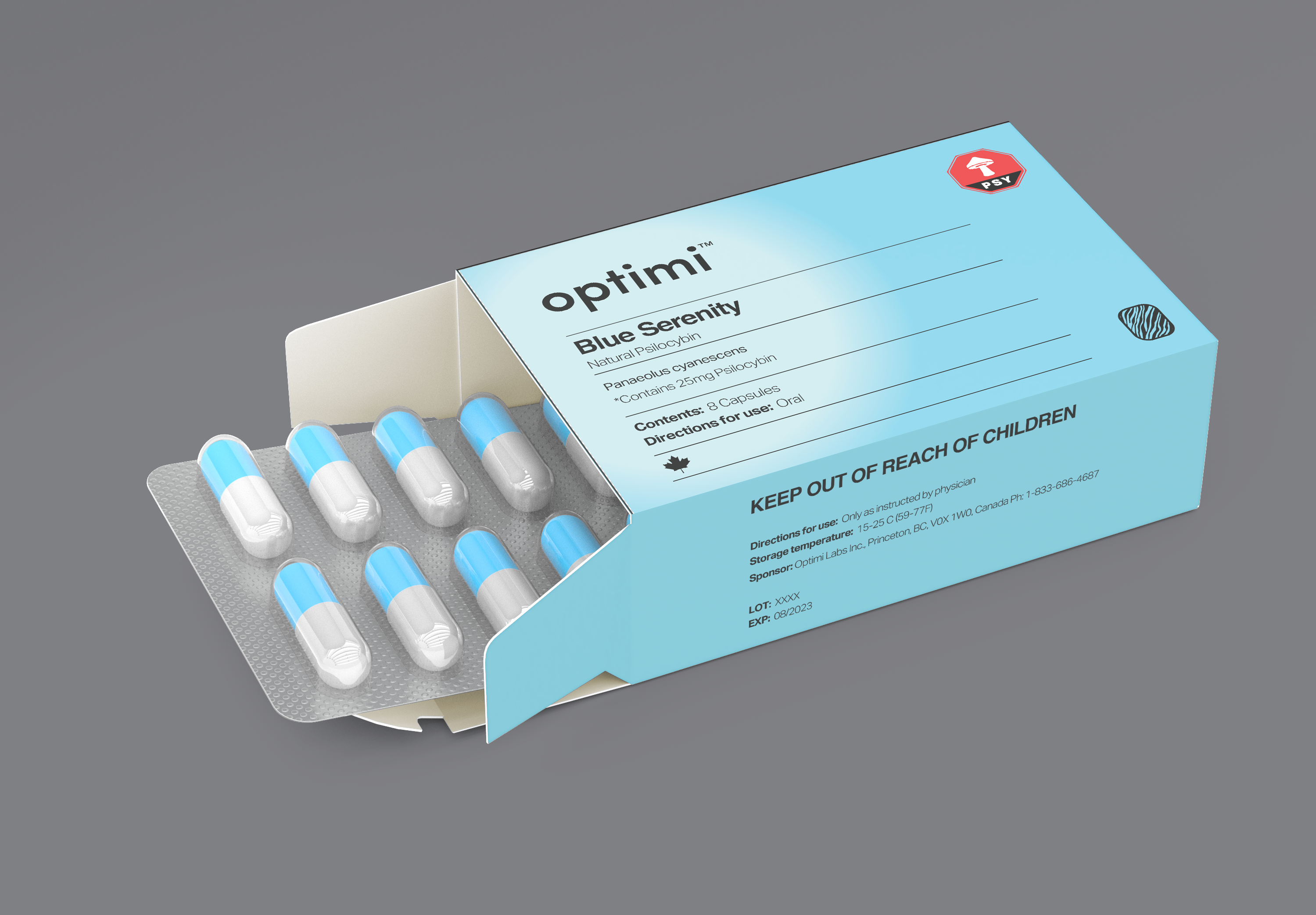
Blue Serenity is packaged according to current regulations and guidelines governing the manufacture and commercial distribution of medical/pharmaceutical products (EU-GMP).
Optimi Health announces the launch of a psilocybin product
VANCOUVER — Optimi Health Corp., a Canadian-based company licensed by Health Canada to produce natural, scalable, and accessible psychedelic and functional mushrooms announces the launch of Blue Serenity, a natural therapeutic psilocybin product, in collaboration with psilocybin patient advocate Thomas Hartle.
Blue Serenity is standardized to contain a total of 25 milligrams of natural psilocybin in the form of EU-GMP biomass grown as Panaeolus cyanescens mushrooms cultivated at Optimi Labs Inc. in Princeton, British Columbia. Optimi’s Chief Science Officer, Justin Kirkland, and Head of Cultivation, Todd Henderson, collaborated with Hartle to breed a specific genetic strain based on Hartle’s previous therapeutic experiences.
Once approved by health authorities under Canada’s Special Access Program (SAP) or an authorized clinical trial, patients, doctors, and researchers will receive Blue Serenity in blister packs of eight (8) psilocybin capsules. Blue Serenity is packaged according to current regulations and guidelines governing the manufacture and commercial distribution of medical/pharmaceutical products (EU-GMP).
Optimi CEO, Bill Ciprick, says:
“Anyone who has entered the psychedelics space in the last two years will tell you that Thomas Hartle’s story of compassion and kindness inspired them in some way to want to make a difference,” said Ciprick. “We are tremendously excited to be partnering with Thomas to become the first Canadian company to launch a natural psilocybin product specifically to help patients seeking relief through the Special Access Program.”
Research shows that up to 80 percent of patients with advanced cancer are likely to suffer distressing thoughts around death and up to 50 percent of patients with generally incurable conditions are likely to have psychiatric diagnoses.
Blue Serenity will be available upon request to all legal psilocybin patients whose access has been approved by Health Canada under legal mechanisms such as the SAP and Section 56 Exemptions to the Controlled Drugs and Substances Act, as well as authorized researchers interested in standardized dosages of natural psilocybin.