
PharmaTher advancing research for novel microneedle delivery of ketamine
by CM Staff
Represents a potential next-generation treatment for neuropsychiatric, neurodegenerative and pain disorders
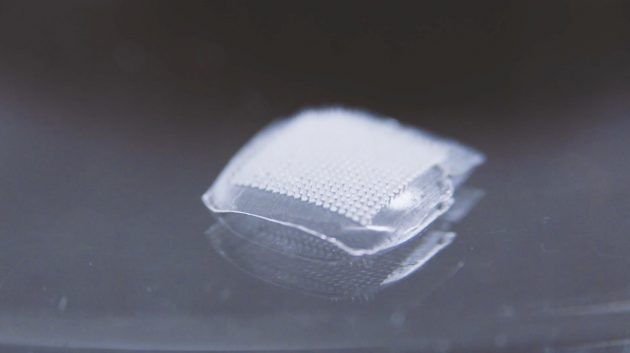
Microneedle Patch Prototype (CNW Group/Newscope Capital Corporation)
TORONTO — Newscope Capital Corporation announced that its wholly-owned subsidiary, PharmaTher Inc., a psychedelics biotech company, has entered into a sponsored research agreement with The Queen’s University of Belfast for the development of a patented hydrogel-forming microneedle patch to deliver ketamine and the PharmaTher’s proprietary ketamine formulation, KETABET. This advance represents a potential next-generation treatment for neuropsychiatric, neurodegenerative and pain disorders.
The research will be led by Professor Ryan Donnelly. Most recently, Donnelly’s lab completed research and published a paper titled Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery.
The proposed ketamine and KETABET MN patch offer a therapeutic solution for various unmet medical needs. Ketamine is becoming an emerging treatment option for major depressive disorder, bipolar depression3, depression with suicidal ideation4 and post-traumatic stress disorder. Despite its potential, ketamine has the potential for abuse and misuse. These risks have led to its limited clinical use and discontinuation.
KETABET has shown in clinical research to enhance the antidepressant effect while having the potential to significantly reduce the known negative side effects of ketamine.
PharmaTher’s patented MN technology consists of hydrogel-forming microneedle arrays and accompanying reservoir which will overcome any limitations by the quantity of drug that can be loaded into the needles or onto the needle surfaces. As such, the MN technology can greatly increase the amount of drug that can permeate through the microneedle array and into the skin8.
PharmaTher’s KETABET MN patch aims to empower patients to dose their medication remotely, safely and conveniently rather than being under supervision by a healthcare provider at a certified medical office. KETABET™ MN patch has the potential for enabling continuous delivery of KETABET (without pain) with minimal formulation manipulation into systemic circulation while maintaining constant plasma levels for more than 24 hours that will improve efficacy and compliance for patients.
“The potential for ketamine is significant and we are leading the way to develop a better ketamine solution to treat these unmet medical needs,” said Fabio Chianelli, CEO of PharmaTher, in a prepared statement. “We are pursuing the clinical development of KETABET MN patch to overcome the current limitations of ketamine and to unlock the known potential therapeutic value of ketamine as a prescription for regulatory approval worldwide. We look forward to working with Professor Donnelly in delivering the next generation ketamine solution.”
Professor Ryan Donnelly commented, “Our lab has successfully delivered esketamine using our patented microneedle technology, which shows the potential of an alternative delivery method that can overcome the limitations of current ketamine delivery options without comprising the safety and compliance of patients. We are excited to work with PharmaTher in their quest to develop a next generation ketamine solution that could help the millions of people who suffer from mental health worldwide.”