
Health Canada certifies Winnipeg Ventilator 2.0 for immediate distribution
by CM Staff
StarFish presented the Ventilator design to expert review panels convened by NGen and ISED
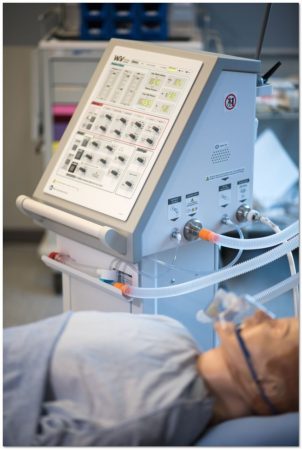
PHOTO: StarFish Medical
VICTORIA — StarFish Medical, a Canadian medical device design, development, and manufacturing service provider, announced Sept. 25 that the Winnipeg Ventilator 2.0 has received a Health Canada COVID-19 Medical Device Authorization under the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19 made by the Minister of Health on March 18, 2020.
The approval allows Canadian Emergency Ventilators Inc. (CEV), as the manufacturer of record, to ship ventilator units to the Public Health Authority of Canada, starting immediately.
“Our goal with the Winnipeg Ventilator 2.0 is to deliver a fully featured ICU ventilator that could save patients’ lives, be manufactured in Canada in the shortest time possible, and not disrupt the supply chain for existing ventilators”, said StarFish Medical CEO and Founder, Scott Phillips in a prepared statement. “To do that, we started with proven technology (original Winnipeg Ventilator designed by Dr. Magdy Younes), updated the design to incorporate technical advances and use non-medical supplier components, all while drawing upon a network of companies we have worked with for over 20 years. The pioneering work of Dr. Younes, and the support of Cerebra Health with clinical input and upcoming clinical trials is invaluable.”
StarFish presented the Ventilator design to expert review panels convened by NGen and ISED.
“StarFish Medical stepped up early on with their commitment to produce a made-in-Canada ventilator design that will help save the lives of COVID-19 patients battling the disease,” said Navdeep Bains, minister of Innovation, Science and Industry. “I am glad we were able to support StarFish Medical in producing this important lifesaving equipment.”
“The Canadian Pandemic Ventilator project led by StarFish Medical is a shining example of how Canadian companies have responded rapidly to the COVID-19 crisis, combining their engineering, technology, and manufacturing capabilities to develop and produce an innovative solution that will help save lives,” said Jayson Myers, NGen’s CEO. “Health Canada’s approval will now let the project partners move into manufacturing and get their ventilator into the hands of the patients who need them most.”