
Health Canada approves Lunit’s AI breast cancer screening tool
by CM Staff
Canada's medical device market is expected to grow from about $7.2 billion in 2019 to $9.2 billion in 2024, with an annual average growth rate of 4.6 per cent.
Lunit INSIGHT CXR
SEOUL — Lunit has received commercial approval in Canada for Lunit INSIGHT, the company’s AI solution suite for radiology.
On June 14, Health Canada issued class 2 medical licenses for Lunit’s AI solution for chest x-ray, ‘Lunit INSIGHT CXR’ and ‘Lunit INSIGHT MMG,’ AI solution for mammography.
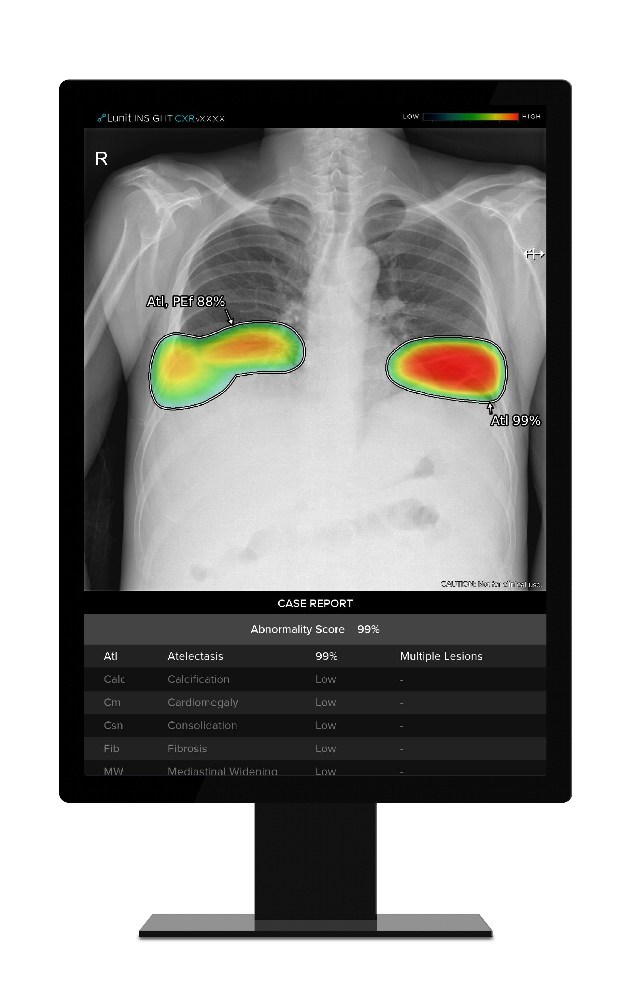
Lunit INSIGHT CXR detects suspicious lesions in chest x-ray images, helping radiologists distinguish suspected disease areas by providing the location of the lesion with an abnormality score that reflects the AI’s confidence level.
Lunit says it has trained this device with over 3,500,000 clinically proven data sets. The company’s INSIGHT CXR can detect 10 of the most common chest abnormalities, including supporting tuberculosis screening, with 97 to 99 per cent accuracy according to the company’s announcement.
The company’s announcement adds that Lunit INSIGHT MMG is one of its mature radiology products, analyzing mammography images with high speed and 96 per cent accuracy. The product has reportedly shown to reduce the chances of undetected breast cancer cases by 50 per cent in mammogram screenings.
Following last year’s FDA approval in the U.S., Lunit’s commercial approval in Canada signals the company’s continued expansion in the North American market.
The company says its own research has indicated that the Canadian medical device market will grow along with the country’s aging population.
Accordingly, the medical device market in Canada is expected to grow from about $7.2 billion in 2019 to $9.2 billion in 2024, with an annual average growth rate expected to be 4.6 per cent.
“This is an important milestone for Lunit, especially after our FDA clearance. North America constitutes the biggest proportion of the global medical device market, and we will make a full-fledged entry following our Canadian and U.S. commercial approval,” said Brandon Suh, CEO of Lunit in a statement.